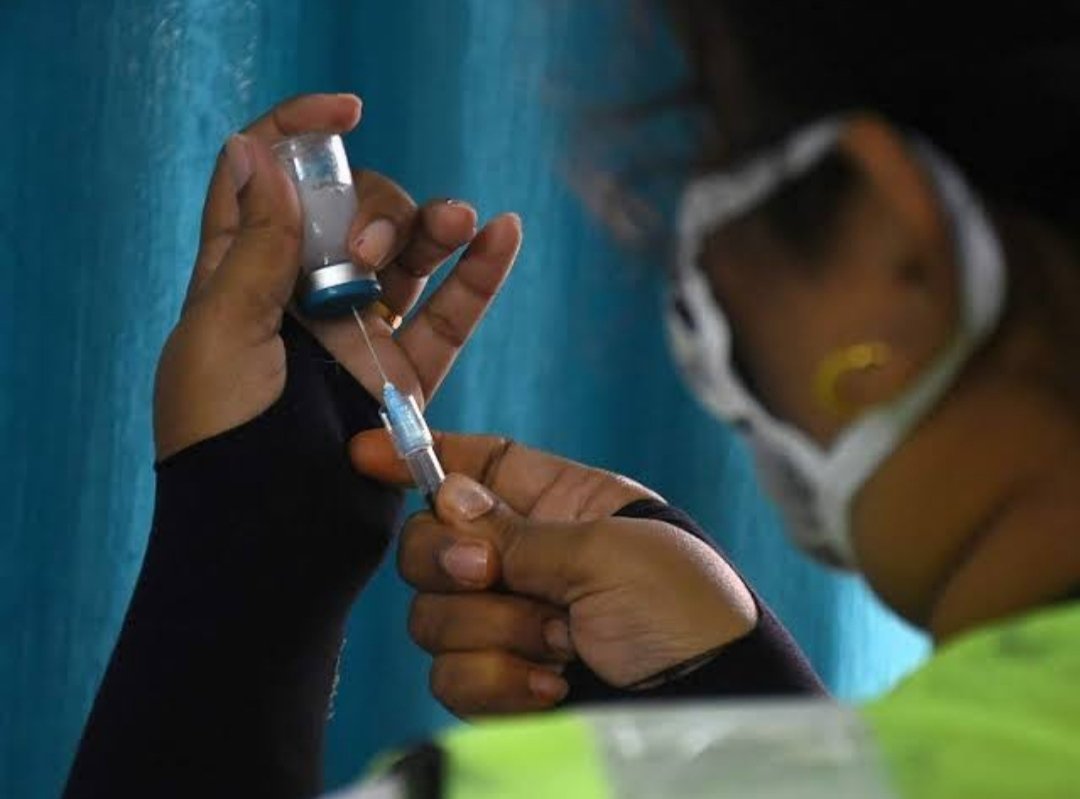
Keypoints:
- With the ramping up of domestic production and arrival of foreign vaccines, availability of vaccine in India will improve by July, said a top official.
- Production of Covaxin and Covishield is being scaled up.
- Russian made Sputnik-V vaccine is also likely to be available in India after May.
Dr. Vinod Paul, who is a NITI Aayog member and also the chairman of government’s empowered group on Covid management, has said that relaxation of norms will let the country’s fast-track greenlighting of foreign-made vaccines that have been granted emergency-use approvals in other countries. He also said that the production of Covaxin and Covishield is being scaled up.
Vaccine availability will be improved by July, with the ramping up of domestic production and arrival of foreign vaccines.
“We are expecting current production levels of Covaxin and Covishield to be scaled up, as has been committed by their manufacturers (Bharat Biotech and Serum Institute). We also expect Pfizer, Moderna and Johnson & Johnson to seek Indian licence as soon as possible,” Dr Paul said.
Sputnik-V, which is a Russian made vaccine, will be available in India after May.
A few of the foreign made vaccines require very low storage temperature-from -25°C to -70°C. Dr Paul said, depending on approvals, some of these shots can be deployed in urban centres.
It has been recommended by the National Expert Group on Vaccine Administration that the vaccines for Covid-19 which have been developed and manufactured in foreign countries, may be granted approvals in India. Those approvals will be accompanied by relevant checks.
“The first 100 beneficiaries of these foreign vaccines will be assessed for a week for safety outcomes, before they are rolled out as part of the larger immunization programme in the country,” Dr Paul said.
“Better the availability, the more age groups we can cover. The government is looking to first cover the 45-plus group and then, depending on availability, expand the drive to cover others, which has been the demand of many states,” he added.
Dr. Paul also said that while more Covishield doses are expected, the supply of Covaxin will see considerable improvement in the future followed by production approvals to public sector units.